💦 FULL SET: Gallery - Collection
Review
doi: 10.1083/jcb.200607083.
Epub 2006 Oct 16.
Membranes of the world unite!
Affiliations
- PMID: 17043140
- PMCID: PMC2064561
- DOI: 10.1083/jcb.200607083
Item in Clipboard
Review
Membranes of the world unite!
J Cell Biol.
.
Abstract
Despite diverse origins, cellular fusion mechanisms converge at a pathway of phospholipid bilayer fusion. In this mini-review, we discuss how proteins can mediate each of the three major stages in the fusion pathway: contact, hemifusion, and the opening of an expanding fusion pore.
Figures

Membrane fusion through hemifusion intermediates. At the state of initial contact (A), lipid bilayers of biological membranes are covered by membrane proteins (pink shapes) including, among others, proteins that mediate membrane binding and fusion. Membrane-associated proteins move apart to allow local close contact between two membrane bilayers (B) and a merger of their contacting leaflets into a stalklike hemifusion connection (C) that expands into a small HD (D). A lipidic fusion pore opens in a HD (E). This pore gives rise to an hourglass fusion pore (F), expansion of which completes the fusion reaction. Blue lines show the bilayer surfaces formed by lipid polar heads. When present in contacting membrane leaflets, inverted cone–shaped lipids such as LPC (shown in green) do not fit into the curvature of the lipid monolayer forming a stalk intermediate (C) and inhibit hemifusion. When added to distal leaflets, the same lipid fits the curvature of the fusion pore edge (E) and promotes pore opening.
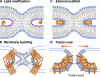
Mechanisms by which fusion proteins might promote hemifusion and fusion pore development. (A) Fusion proteins (shown as orange shapes) might change local lipid composition by generating fusogenic lipids (shown in pink) of contacting leaflets of the fusing membranes to that promoting hemifusion. Lipid-modifying enzymes might also change the composition of distal leaflets to that promoting pore development (not shown). (B) Folding of fusion proteins (shown as the release of an extended spring) might drive hemifusion and fusion by producing bending stresses in bilayers bulged toward each other. (C) Lipids might be scaffolded onto surfaces of fusion proteins. For instance, protein scaffold located outside the hemifusion connection might present a positively charged electrostatic surface that would bind negatively charged lipids, and facilitate hemifusion and provide a handle for “pulling” the stalk open. (D) Proteins might develop a dense interconnected protein coat around the fusion site. Because of protein–protein interactions and protein shape, the protein coat has an intrinsic curvature (shown here as springs). This coat “wants” to deform the underlying lipid bilayers, thus, producing the lateral tension that drives the transition from hemifusion to opening an expanding fusion pore.
References
-
- Barona, T., R.D. Byrne, T.R. Pettitt, M.J. Wakelam, B. Larijani, and D. Poccia. 2005. Diacylclycerol induces fusion of nuclear envelope membrane precursor vesicles. J. Biol. Chem. 280:41171–41177. - PubMed
-
- Chernomordik, L.V., and M.M. Kozlov. 2003. Protein-lipid interplay in fusion and fission of biological membranes. Annu. Rev. Biochem. 72:175–207. - PubMed
-
- Chernomordik, L.V., and M.M. Kozlov. 2005. Membrane hemifusion: crossing a chasm in two leaps. Cell. 123:375–382. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

